Demystifying the Difference: Unit Operations vs Unit Processes – A Clear Distinction:
 Anand Rode
Anand Rode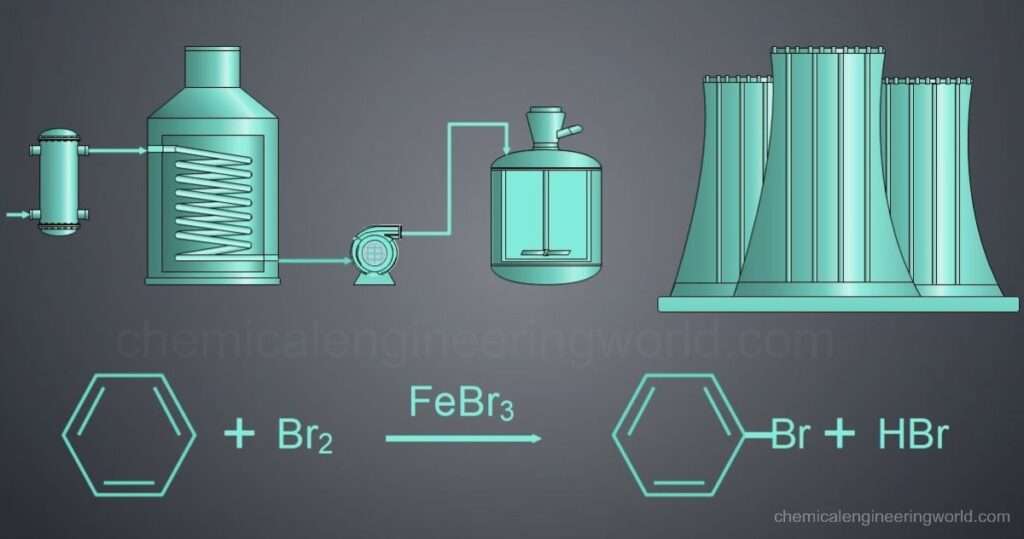
Unit Operation and Unit Process :- The entire chemical engineering can be classified into two groups; unit operations or unit processes. The concept of unit operations was introduced in 1915 by Dr. Arthur D. Little. The concept of unit processes was introduced in 1923 by P.H. Groggin.
The world of chemical engineering might seem like a labyrinth of bubbling concoctions and intricate machinery. But fear not, for within this intriguing realm lie fundamental building blocks called unit operations and unit processes. These terms are often used interchangeably, but understanding the subtle distinction between them is key to appreciating the magic behind the processes that create everything from your favorite beverage to life-saving medications.
Unit Operations: The Masters of Physical Transformation
Imagine a sculptor meticulously shaping a lump of clay. Unit operations function in a similar way, but on a much smaller, molecular level. They deal with physical changes in materials, manipulating their state, size, or composition without altering their chemical identity. Here's a breakdown to illustrate the concept:
Separation Techniques: Distillation, a prime example, separates mixtures based on boiling points. Filtration removes solid particles from a liquid, while extraction selectively pulls out desired components using a solvent.
Heat and Mass Transfer: These operations focus on the movement of heat or mass between phases. Think of evaporation concentrating a solution by removing solvent through vaporization. Conversely, condensation transforms vapor back into liquid form. Crystallization isolates a solid solute from a solution by promoting crystal formation.
Size Reduction and Mixing: Grinding, crushing, and milling break down larger particles into smaller ones. Mixing, on the other hand, combines different materials to achieve uniformity.
Unit Processes: Orchestrating Chemical Transformations
Now, let's shift gears to the fascinating realm of chemistry. Unit processes are all about inducing chemical reactions, where the fundamental structure of the molecules themselves undergoes change.
Reaction Engineering: This involves optimizing reaction conditions like temperature, pressure, and catalyst usage to maximize product yield, selectivity, and reaction rate. Examples include fermentation, where sugars are converted to alcohol, and polymerization, where monomers link to form long polymer chains.
Stoichiometry: This branch of chemistry helps us determine the ideal ratios of reactants required for a complete reaction, ensuring efficient use of raw materials.
The Symphony of Unit Operations and Unit Processes
In the real world, chemical processes rarely rely solely on unit operations or unit processes. They are often intricately linked:
Setting the Stage for Reactions: Unit operations often prepare materials for subsequent chemical reactions. For instance, distillation might purify a reactant stream before it enters a reactor.
Refining the Products: After a reaction, unit operations may be used to isolate and purify the desired product. Crystallization can isolate a specific product from a reaction mixture, while distillation can further purify it.
By understanding and combining these fundamental building blocks, chemical engineers design and optimize complex industrial processes that produce everything from life-saving drugs to the plastics that make our world function. So, the next time you marvel at a new material or appreciate the purity of your drinking water, remember the silent symphony of unit operations and unit processes playing a vital role behind the scenes.
This blog post has hopefully shed light on the distinction between unit operations and unit processes. Now, you're equipped to delve deeper into the fascinating world of chemical engineering, where these fundamental principles orchestrate the transformations that shape our world!
Subscribe to my newsletter
Read articles from Anand Rode directly inside your inbox. Subscribe to the newsletter, and don't miss out.
Written by
