The Floating Phenomenon: Unraveling the Mystery of Why Ice Floats on Water
 Quantum Cyber Solutions
Quantum Cyber Solutions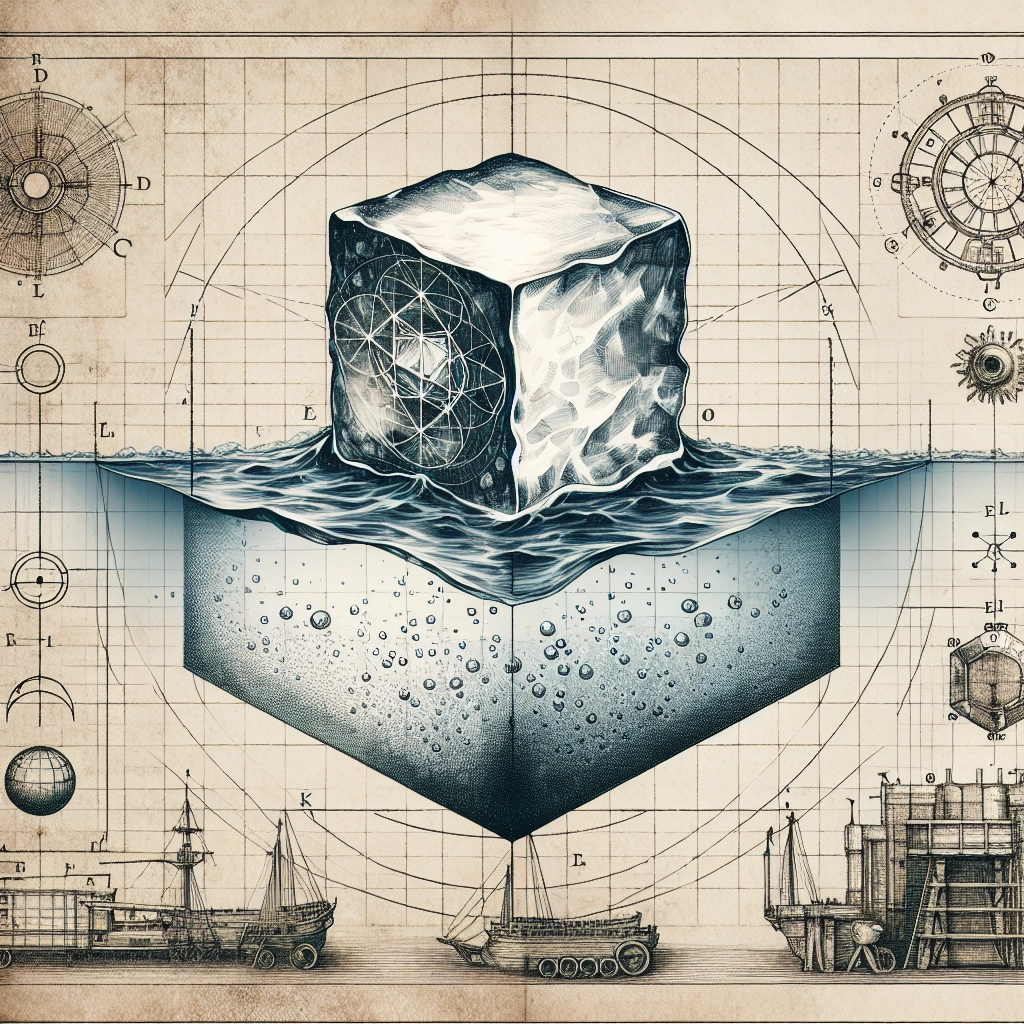
Published on
Friday, May 29, 2020
The Floating Phenomenon: Unraveling the Mystery of Why Ice Floats on Water
==============================================================================
Authors

Name
Eric deQuevedo 😄
Twitter
❄️ The Paradox of Floating Ice: A Cool Conundrum ❄️
Picture this: you're enjoying a refreshing glass of iced water on a hot summer day. As you watch the ice cubes bobbing on the surface, you might find yourself wondering, "Why does ice float on water?" After all, most solids are denser than their liquid counterparts and tend to sink. So, what makes ice the exception to this rule? In this blog post, we'll unravel the mystery of floating ice and explore the fascinating science behind this seemingly paradoxical phenomenon.
🌊 The Density Dilemma: Understanding the Basics 🌊
To understand why ice floats on water, we first need to grasp the concept of density. Density is a measure of how much mass an object has per unit of volume. In simpler terms, it's a way to describe how tightly packed the particles within a substance are.
In most cases, solids are denser than liquids because their particles are more closely packed together. When a solid object is placed in a liquid, it will sink if its density is greater than that of the liquid. Conversely, if the solid is less dense than the liquid, it will float.
So, why does ice, a solid, have a lower density than liquid water? The answer lies in the unique properties of water molecules and the way they behave as water transitions from liquid to solid state.
🔬 The Molecular Dance: Hydrogen Bonding in Water 🔬
Water is an extraordinary substance with many anomalous properties, and its behavior during the freezing process is no exception. The secret to ice's buoyancy lies in the way water molecules interact with each other through hydrogen bonding.
In liquid water, molecules are constantly moving and forming temporary hydrogen bonds with neighboring molecules. These bonds are continually breaking and reforming, allowing water to flow and adapt to its container.
However, as water cools and approaches its freezing point, the molecules begin to slow down and vibrate less. This allows the hydrogen bonds to become more stable and persistent, causing the molecules to arrange themselves in a highly organized, crystal-like structure.
⚖️ The Density Difference: Ice vs. Liquid Water ⚖️
The key to ice's lower density lies in the geometry of its crystalline structure. When water freezes, the molecules align in a hexagonal pattern, creating an open, cage-like arrangement with more space between the molecules compared to liquid water.
This increased space between the molecules in ice results in a lower density than liquid water. At 0°C (32°F), the density of ice is about 0.9167 grams per cubic centimeter, while the density of liquid water at the same temperature is about 0.9998 grams per cubic centimeter.
It's this small but significant difference in density that allows ice to float on water. When an ice cube is placed in a glass of water, it displaces an amount of water equal to its weight. Since the ice is less dense than the water it displaces, it floats on the surface.
🌡️ The Importance of Ice's Buoyancy: Ecological and Practical Implications 🌡️
The fact that ice floats on water might seem like a simple curiosity, but it has profound implications for life on Earth and our daily experiences.
From an ecological perspective, ice's buoyancy plays a crucial role in the survival of aquatic life in cold regions. When lakes and rivers freeze, the ice forms a protective layer on the surface, insulating the water below and allowing aquatic life to survive the harsh winter months. If ice were denser than water and sank, entire bodies of water could freeze solid, making it impossible for many organisms to survive.
In our everyday lives, ice's buoyancy is what keeps our drinks cool and refreshing. As ice cubes float on the surface of our beverages, they slowly melt and cool the liquid around them, creating a delightful temperature gradient that we can enjoy sip after sip.
🧊 The Science of Ice: Beyond Floating 🧊
While the floating property of ice is undoubtedly fascinating, it's just one of the many intriguing aspects of this remarkable substance. Ice exhibits a range of unique characteristics that have captivated scientists for centuries:
Regelation: The ability of ice to melt under pressure and refreeze when the pressure is released, allowing ice to flow and behave plastically.
Expansion Upon Freezing: Unlike most substances, water expands as it freezes, which is why ice is less dense than liquid water. This expansion can cause pipes to burst during cold weather.
Multiple Crystalline Forms: Depending on the pressure and temperature conditions, water can freeze into several different crystalline structures, each with its own unique properties.
Supercooling: Under certain conditions, water can remain liquid even below its normal freezing point, a state known as supercooling. When disturbed, supercooled water can freeze almost instantly.
These fascinating properties of ice have inspired countless scientific studies and have far-reaching implications in fields ranging from glaciology to cryobiology.
🌟 Embracing the Wonder of Floating Ice 🌟
The next time you find yourself mesmerized by the sight of ice cubes floating in your drink, take a moment to appreciate the remarkable science behind this everyday occurrence. The floating of ice on water is a testament to the extraordinary properties of water and the intricate dance of molecules that make it possible.
So, when someone asks you, "Why does ice float on water?" you can confidently share the story of hydrogen bonding, crystalline structures, and the density difference that allows this solid to defy expectations and float gracefully on the surface.
Embrace the wonder of floating ice and let it serve as a reminder of the fascinating science that underlies even the most seemingly simple aspects of our world. From the ecological importance of ice's buoyancy to the practical joys of a perfectly chilled drink, the floating phenomenon of ice is a cool conundrum that never fails to captivate and inspire.
Discuss on Twitter • View on GitHub
Tags
Ice
Water
Density
Hydrogen Bonding
Anomalous Properties
Questions
Previous Article
The Skys Blue Hue: Unraveling the Mystery and Exploring Other Worlds
Next Article
The Popcorn Phenomenon: Exploring the Science Behind the Pop
Subscribe to my newsletter
Read articles from Quantum Cyber Solutions directly inside your inbox. Subscribe to the newsletter, and don't miss out.
Written by
