The Tragic Story of the Dalkon Shield Contraceptive
 Chinonye Ruth Ibeakanma
Chinonye Ruth Ibeakanma
As a Biomedical Engineering student currently taking a course in medical software, I’m learning firsthand how crucial patient safety is in the design and development of medical devices. The Dalkon Shield, a contraceptive device introduced in the 1970s, is a powerful example of what can go wrong when thorough testing and ethical considerations are neglected.
This story is not just a historical footnote but a critical lesson for future engineers like myself. It highlights the critical need for thorough design and strong regulatory oversight to ensure that innovations genuinely serve and protect patients.
Imagine a world where a contraceptive device, designed to empower women with control over their reproductive health, instead leads to widespread injury and suffering. The Dalkon Shield, introduced in the early 1970s and initially celebrated as a breakthrough in birth control, soon became infamous for its detrimental effects on women's health.

The Context: Contraceptive Options in the 1970s
During the early 1970s, contraceptive options were limited and those available were either ineffective or cumbersome. Barrier methods, such as condoms or diaphragms, were popular but had varying effectiveness and required consistent use. Hormonal methods, including the birth control pill, were becoming more popular but were accompanied by a range of side effects.
Within the context of limited and imperfect choices, the Dalkon Shield was introduced with high expectations. The introduction of the Dalkon Shield was highly anticipated as it offered the promise of a more reliable and convenient contraceptive option. It was seen as a promising solution for women seeking a more effective and less demanding method of birth control.
Design and Development of the Dalkon Shield
The Dalkon Shield, introduced in the early 1970s, was an advanced contraceptive device made of plastic with a polyvinyl acetate core, featuring multiple outward-extending arms. This distinctive shape was designed to conform to the uterus, ensuring a secure fit and increased endometrial contact to enhance contraceptive effectiveness.
The device also included copper, which acted as a spermicide to further boost its efficacy. Marketed as a "set-it-and-forget-it" solution, the Dalkon Shield aimed to provide a reliable and hassle-free alternative to other birth control methods that were often less effective or more cumbersome.
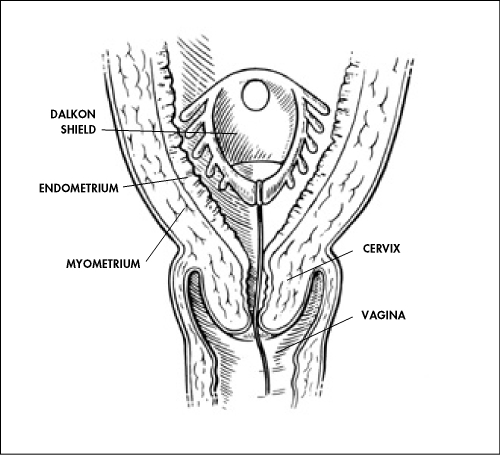
Health Impact and Public Outcry
The Dalkon Shield’s design included a multifilament removal string intended to facilitate its extraction. However, patients reported that this string interfered with intercourse, causing discomfort and irritation. In response to these complaints, the manufacturer, AH Robins, replaced the multifilament string with a single-strand twine.
This modification, however, worsened the situation, as the twine was inadequately designed to prevent infections and became a conduit for bacteria. As a result, users experienced a range of severe infections, including pelvic inflammatory disease (PID), which could lead to long-term reproductive damage and infertility.
Despite it's intended benefits, the Dalkon Shield has critical flaws. The arms, designed to stabilize the device, frequently caused physical discomfort and irritation. The copper coating, designed to act as a spermicide, failed to address the severe infections associated with the device’s removal string. These problems, along with a lack of thorough testing, resulted in significant health complications and ultimately marred the device’s reputation.
Regulatory Oversight and Response
The approval process for the Dalkon Shield by the Food and Drug Administration (FDA) in the 1970s was significantly less rigorous compared to today's standards. The FDA reviewed the data provided by the manufacturer and allowed the device to be fast-tracked due to its innovative design and the urgent demand for new contraceptive solutions. However, the clinical trials conducted were not thorough enough to uncover critical risks linked to the device’s design and materials, leading to its widespread distribution despite significant health concerns.
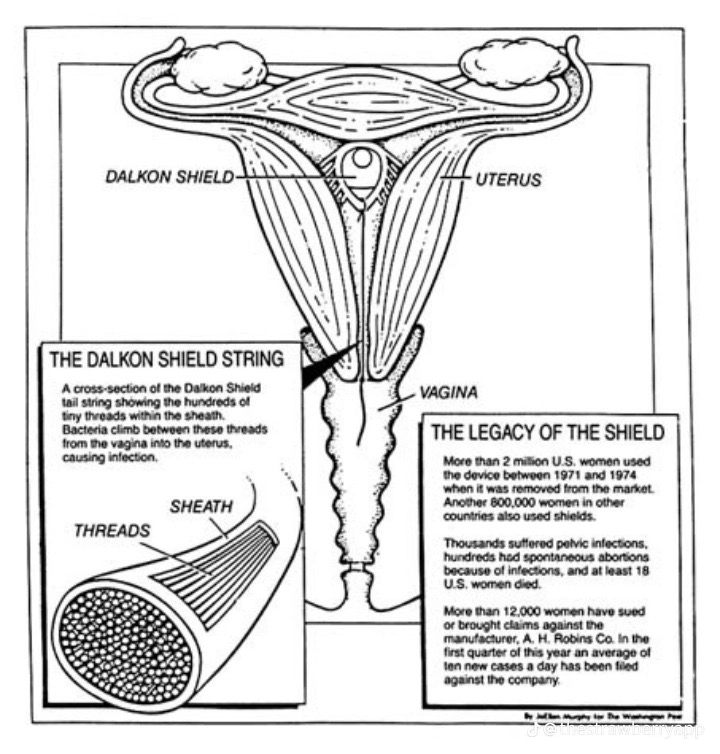
Studies and reports from the time revealed that at least 200,000 women were affected by the device’s adverse effects. Many experienced debilitating conditions such as pelvic inflammatory disease (PID), which could lead to long-term reproductive issues and hospitalizations. The emotional and physical toll on these women was profound, leading to lawsuits and widespread outrage, which further underscored the urgent need for more rigorous testing and regulation of medical devices.
AIn 1974, the FDA took action by requesting A.H. Robins to cease sales of the Dalkon Shield due to growing safety concerns. Nevertheless, the company continued its sales and distribution, driven by financial interests and ongoing consumer demand. It wasn’t until 1980—six years after the FDA’s request—that A.H. Robins finally halted the production of the device. This prolonged delay caused further health complications for many women, highlighting a major lapse in regulatory enforcement and ethical responsibility.
Investigations and Findings
The Dalkon Shield’s safety issues prompted an extensive investigation, driven by alarming health reports from affected women. In 1974, Dr. David E. Smith’s groundbreaking report, published in the New England Journal of Medicine, exposed a troubling link between the Dalkon Shield and severe reproductive health problems, such as pelvic inflammatory disease (PID).
The Dalkon Shield tragedy was a turning point in medical device regulation, leading to the passage of the Medical Device Amendments of 1976. These amendments mandated a stricter premarket approval process, requiring manufacturers to demonstrate the safety and effectiveness of their products through rigorous testing and clinical trials.
The reforms also included post-market surveillance measures, enabling the FDA to monitor the safety of devices once they were in use. These reforms were crucial in preventing the recurrence of such a public health disaster.
As I continue my studies in medical software, the Dalkon Shield's story serves as a compelling reminder of the critical need for rigorous testing and ethical responsibility in medical device development. This historical case highlights how regulatory vigilance and modern technology can work together to ensure patient safety. The lessons from the Dalkon Shield highlight the importance of prioritizing the well-being of patients in every stage of innovation.
Subscribe to my newsletter
Read articles from Chinonye Ruth Ibeakanma directly inside your inbox. Subscribe to the newsletter, and don't miss out.
Written by
