Article Review: CT free attenuation and Monte Carlo based scatter correction guided quantitative 90Y SPECT imaging for improved dose calculation using deep learning
 Aldo Yang
Aldo Yang3 min read
Objectives
- Developed deep learning (DL) models for CT-free attenuation correction (AC) and Monte Carlo-based scatter correction (SC) in quantitative 90Y SPECT imaging.
- Trained three separate models for AC, SC, and joint attenuation and scatter correction (ASC) using a modified 3D Swin UNETR architecture.
- Achieved fast inference times (20 seconds per SPECT image on GPU, 6 minutes on CPU) compared to traditional methods (80 minutes on CPU).
- Used dose maps, derived from corrected and uncorrected SPECT images, as input and output for the models, leveraging meaningful dose values (Gy).
- Evaluated model performance extensively at both organ and voxel levels, demonstrating comparable accuracy to a commercial reconstruction tool.
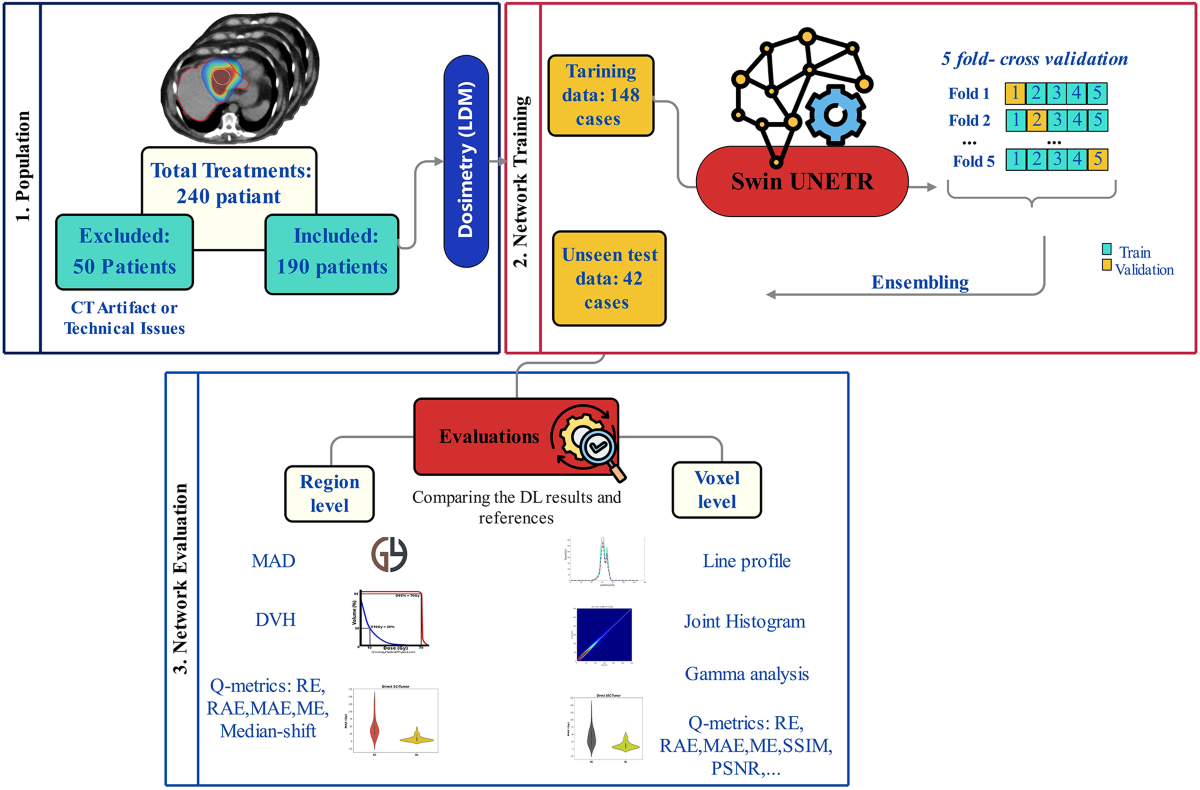
Methodology
- Utilized a modified 3D Swin UNETR architecture, combining a U-shaped network with a Swin Transformer encoder and a Convolutional Neural Network (CNN) decoder.
- Employed a 5-fold cross-validation training scheme on a dataset of 190 patients who underwent 90Y selective internal radiation therapy (SIRT).
- Used random patch sampling (64x64x64 voxels), rotation, and flipping for data augmentation, with dropout set to 0.1.
- Trained models using the Adam optimizer and L1 loss function (Mean Absolute Error) for 200 epochs.
- Performed test image inference using a sliding window method with the same patch size as training.
Results
- Voxel-level quantitative metrics (average±SD) for the AC task: mean error (ME (Gy)): -0.026±0.06, structural similarity index (SSIM (%)): 99.5±0.25, peak signal to noise ratio (PSNR (dB)): 47.28±3.31.
- For the SC task: ME: −0.014±0.05, SSIM: 99.88±0.099, PSNR: 55.9±4.
- For the ASC task: ME: -0.04±0.06, SSIM: 99.57±0.33, PSNR: 47.97±3.6.
- Voxel-level gamma evaluations with criteria “DTA: 4.79, DD: 1%”, “DTA:10 mm, DD: 5%”, and “DTA: 15 mm, DD:10%” yielded pass rates around 98%.
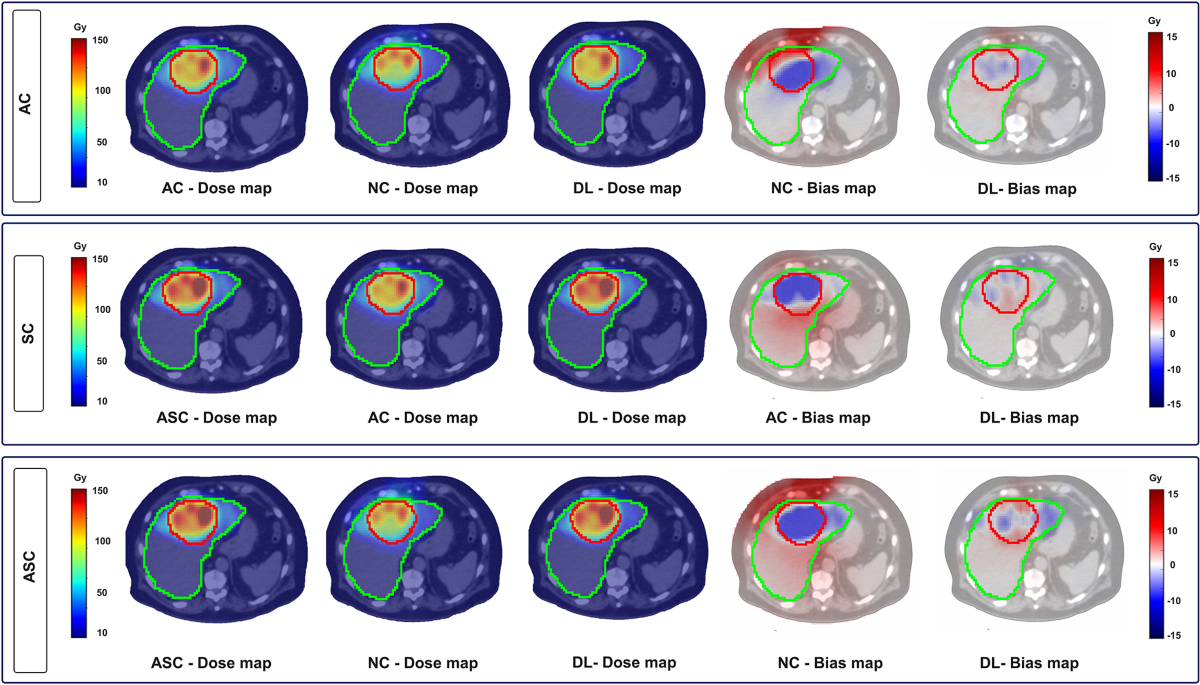
- Mean absolute error (MAE (Gy)) for tumor and whole normal liver (WNL): AC (7.22±5.9 and 1.09±0.86), SC (8±9.3 and 0.9±0.8), and ASC (11.8±12.02 and 1.3±0.98).
Discussions
- The study utilizes a relatively large dataset (190 patients), but it is from a single center, potentially limiting generalizability. Future work should include multi-center data.
- The models' performance is affected by the presence of metal implants and heterogeneous tissue compositions (e.g., calcifications, air-tissue interfaces). The authors should consider incorporating strategies to address these challenges, such as including more diverse training data with these features or developing specific modules to handle them.
- While the study compares the DL models to a commercial reconstruction tool, a more direct comparison with other published deep learning-based methods for AC and SC in 90Y SPECT would strengthen the findings.
- The rationale for using dose maps instead of SPECT images directly is well-justified. However, providing more details on the limitations encountered when training with SPECT images (supplementary material) would be beneficial.
0
Subscribe to my newsletter
Read articles from Aldo Yang directly inside your inbox. Subscribe to the newsletter, and don't miss out.
Written by
