Article Review: Beyond similarities overall survival and prognostic insights from Lu Lu DOTATOC therapy in neuroendocrine tumors
 Aldo Yang
Aldo Yang3 min read
Objectives
This study evaluates the overall survival (OS) and prognostic factors for neuroendocrine tumor (NET) patients treated with [177Lu]Lu-DOTATOC peptide receptor radionuclide therapy (PRRT). Core contributions include:
- Reporting a median OS of 55.2 months in a cohort of 141 NET patients receiving [177Lu]Lu-DOTATOC PRRT, suggesting its effectiveness as a treatment option.

- Identifying baseline De Ritis Ratio, Alkaline Phosphatase (ALP), Chromogranin A (CgA), and prior therapy with mTOR-inhibitors as independent prognostic factors for OS.
- Identifying primary tumor location (Lung-NET) and baseline CgA as independent prognostic factors for a higher risk of primary progression according to RECIST 1.1 criteria.
- Highlighting that [177Lu]Lu-DOTATOC production generates minimal metastable lutetium ([177mLu]), simplifying waste disposal compared to [177Lu]Lu-DOTATATE.
Methodology
This was a monocentric, retrospective analysis of 141 patients with histologically proven, metastasized NET expressing somatostatin receptors (SSR), who received PRRT with [177Lu]Lu-DOTATOC between 2007 and 2021.
- Treatment involved a median of 3 cycles of [177Lu]Lu-DOTATOC (scheduled dose 7.40 GBq/cycle).
- Endpoints included Overall Survival (OS), defined as time from first PRRT cycle to death from any cause, and radiological response assessed by RECIST 1.1 criteria (Overall Response Rate - ORR, Disease Control Rate - DCR, primary progression) within 6 months after the last cycle.
- Statistical analysis employed:
- Kaplan-Meier method for OS estimation.
- Univariable and multivariable Cox proportional hazards regression to identify prognostic factors for OS.
- Univariable and multivariable logistic regression to identify prognostic factors for primary progression.
- Baseline biomarkers evaluated included De Ritis Ratio (AST/ALT), Chromogranin A (CgA, binarized at >204 µg/L), and Alkaline Phosphatase (ALP, binarized at >Upper Limit of Normal - ULN).
Results
The study provides substantial data supporting its claims:
- The median OS for the total cohort (n=141) was 55.2 months (IQR: 27.1–89.7 months). Subgroup OS: SI-NET G1-G2: 62.7 months; P-NET G1-G2: 41.2 months; NET G3: 26.3 months.
- Multivariable Cox regression identified significant independent prognostic factors for shorter OS: De Ritis Ratio (HR: 2.98; p<0.001), ALP > ULN (HR: 2.72; p<0.001) 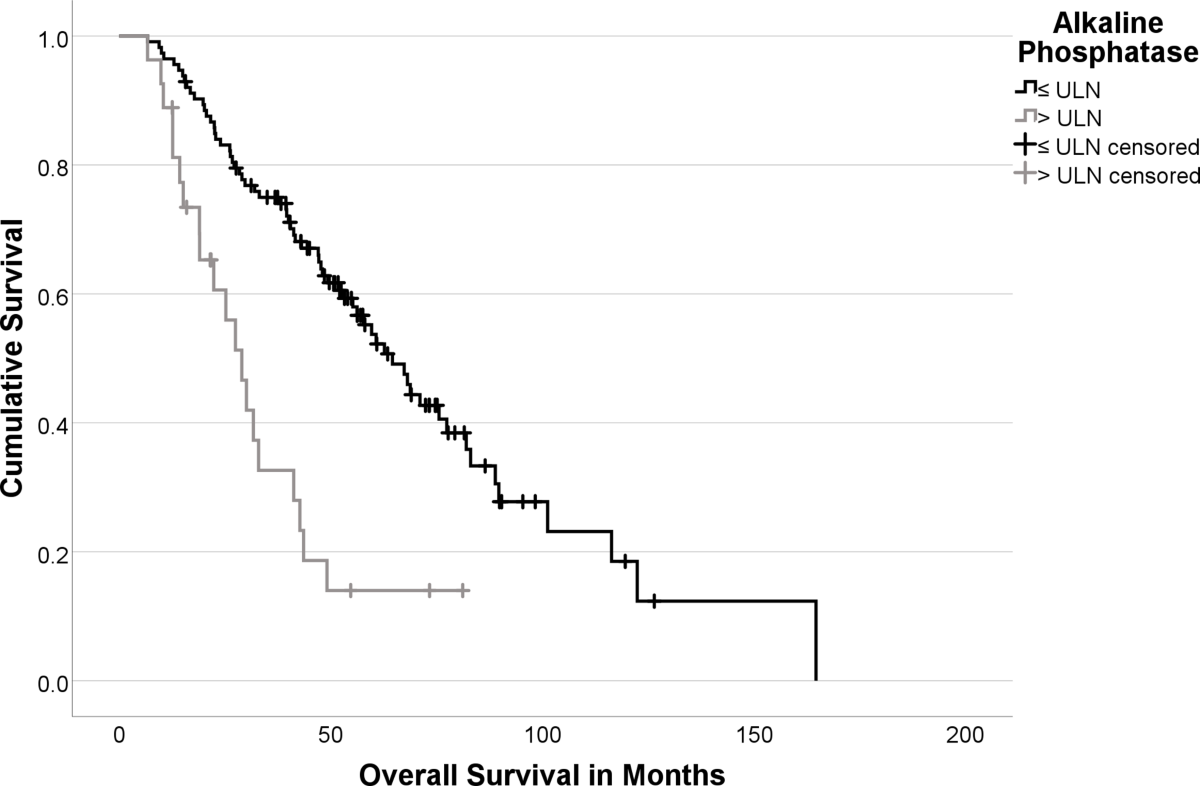, CgA > 204 µg/L (HR: 4.96; p<0.001), and prior mTOR-inhibitor therapy (HR: 2.04; p=0.026).
- Radiological response (n=123): ORR was 12% (11% PR, 1% CR), DCR was 72% (ORR + 60% SD). Primary progression (PD) occurred in 29% of patients.


- Multivariable logistic regression identified significant independent prognostic factors for higher risk of primary progression: Lung-NET vs GI-NET (OR: 4.86; p=0.04) and CgA > 204 µg/L (OR: 4.07; p=0.01).
Discussions
The study provides valuable real-world data on [177Lu]Lu-DOTATOC PRRT outcomes. However, several limitations should be considered:
- The retrospective design introduces potential biases (selection, information). The long inclusion period (2007-2021) spans significant evolution in NET diagnostics (SSR-scintigraphy vs. more sensitive SSR-PET/CT) and potentially therapies, possibly affecting cohort homogeneity and comparability over time, despite consistent Krenning score criteria.
- Heterogeneity in imaging modalities (CT vs. MRI) and follow-up schedules for response assessment could impact the consistency of RECIST 1.1 evaluation.
- The absence of a control group prevents assessment of the identified factors' predictive capability versus purely prognostic significance.
- Variable numbers of PRRT cycles (median 3, range 1-6) due to discontinuation upon PD in interim staging might complicate comparisons with studies using fixed cycle numbers.
- Lack of documented follow-up treatment data after PRRT completion introduces a potential confounding bias on OS.
- A prospective study with standardized imaging protocols, follow-up schedules, and ideally a comparator arm (e.g., [177Lu]Lu-DOTATATE or other standard care) would strengthen the conclusions regarding efficacy and prognostic/predictive factor validation.
0
Subscribe to my newsletter
Read articles from Aldo Yang directly inside your inbox. Subscribe to the newsletter, and don't miss out.
Written by
