Article Review: Fast 4D PET parametric imaging computation at the whole field of view level Reliability under simulated conditions of PET KinetiX a dedicated software solution
 Aldo Yang
Aldo Yang4 min read
Objectives
This study evaluates the reliability of PET KinetiX, a new academic software for fast, whole field-of-view (FOV) 4D-PET parametric imaging computation, using simulated data.
- It demonstrates that PET KinetiX generates Ki and vb parametric maps (using Patlak and two-tissue compartment models, 2TCM) that faithfully reproduce predefined tissue structures from the XCAT digital phantom.
- The generated kinetic maps (Ki, vb) are qualitatively comparable to standard unprocessed Standardized Uptake Value (SUV) data.
- In most simulated tissue structures, kinetic parametric maps, particularly Patlak Ki and vb, showed improved contrast-to-noise ratios (CNR) compared to SUV data.
- The highest CNR enhancement was observed for 2TCM k₃ maps within simulated tumor regions.
Methodology
The study employed simulations to evaluate PET KinetiX reliability.
- 4D-PET data were simulated using the XCAT anthropomorphic digital phantom with realistic 18F-FDG time-activity curves (TACs) derived from non-small cell lung cancer patient data serving as ground truth.
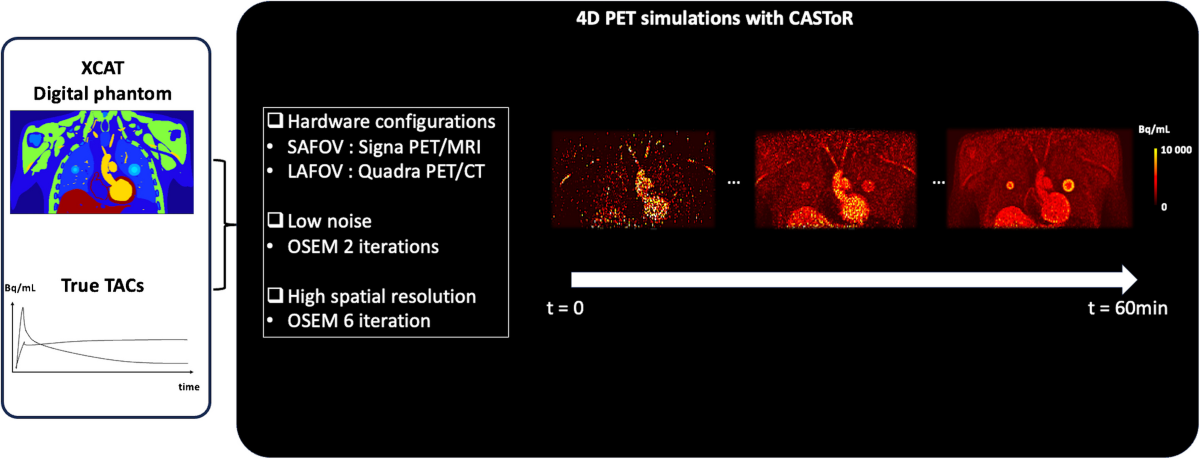
- Simulations replicated two PET system configurations: short axial FOV (SAFOV)-like (GE Signa PET/MR) and long axial FOV (LAFOV)-like (Siemens Vision Quadra), considering differences in sensitivity and time-of-flight capabilities.
- 400 analytical simulations (100 noise realizations for each configuration/iteration setting) were generated and reconstructed using the CASToR open-source platform.
- Reconstructions used the Ordered Subsets Expectation Maximization (OSEM) algorithm with 2 iterations (low noise) and 6 iterations (high spatial resolution), without point spread function modeling or post-filtering.
- PET KinetiX processed the reconstructed 4D-PET data using an indirect method with an image-derived input function (IDIF) from the thoracic aorta.
- Parametric maps were generated for the Patlak model (Ki, vb) and the irreversible 2TCM (K1, k2, k3, vb, Ki).
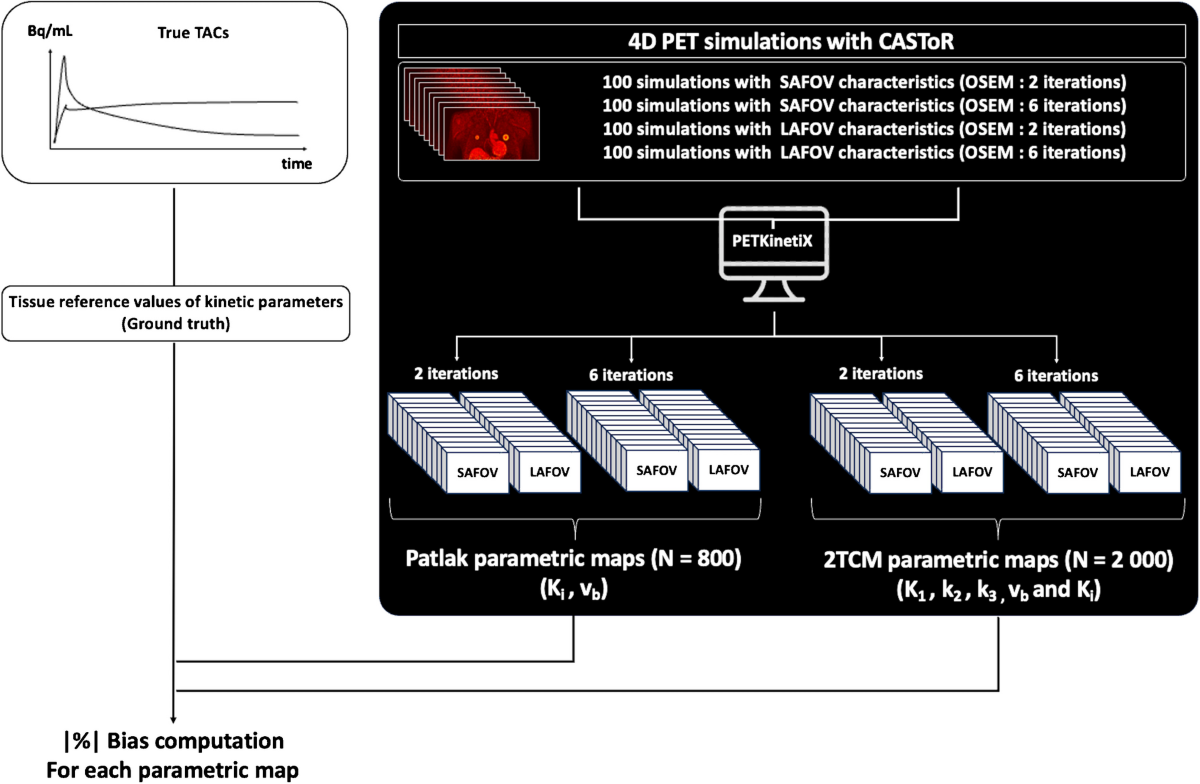
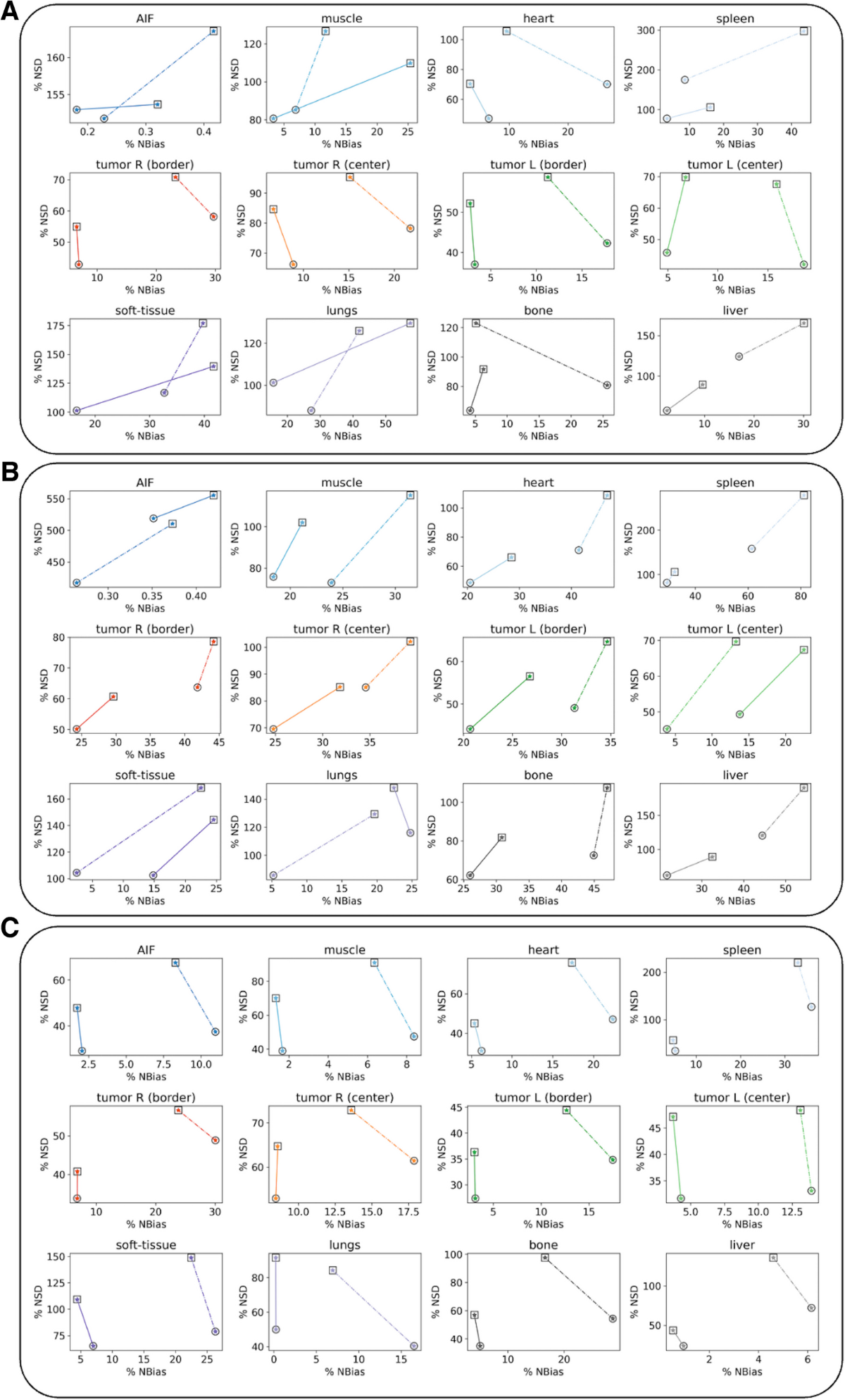
- Evaluation involved calculating mean bias and standard deviation of kinetic parameters compared to ground truth for 11 tissue labels (including tumor center/border, liver, lungs, bone, heart, spleen, muscle, fat).
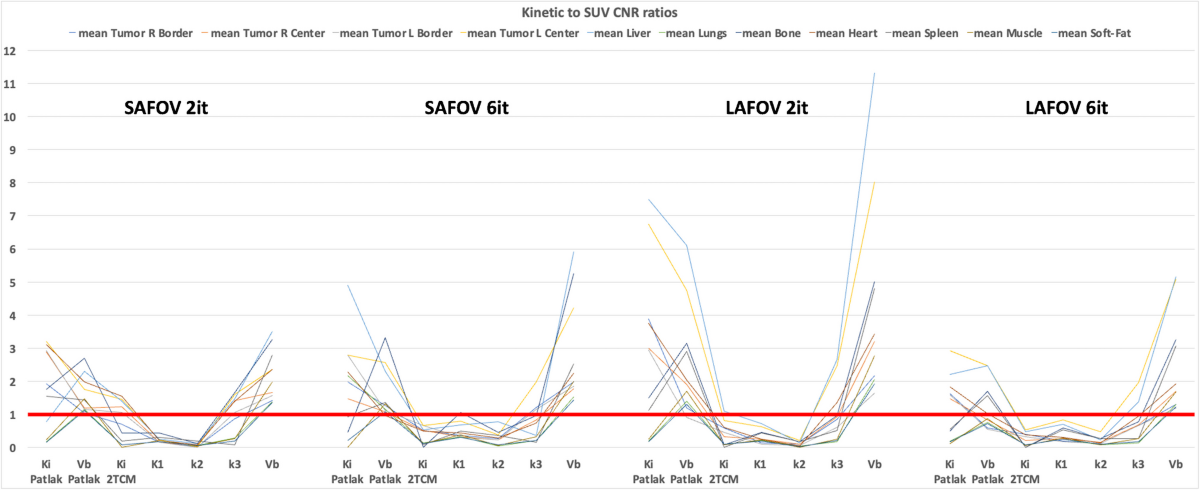
- Contrast-to-noise ratio (CNR) was calculated for each tissue structure relative to the superior vena cava, and kinetic-to-SUV CNR ratios were computed.
Results
The study provides quantitative data to support its claims:
- A total of 2,800 parametric maps (800 Patlak, 2000 2TCM) were generated from 400 simulations.
- Visual quality of Ki and vb maps was high, reproducing phantom structures well, with LAFOV providing sharper definition than SAFOV, and 6 iterations sharper (but noisier) than 2 iterations.
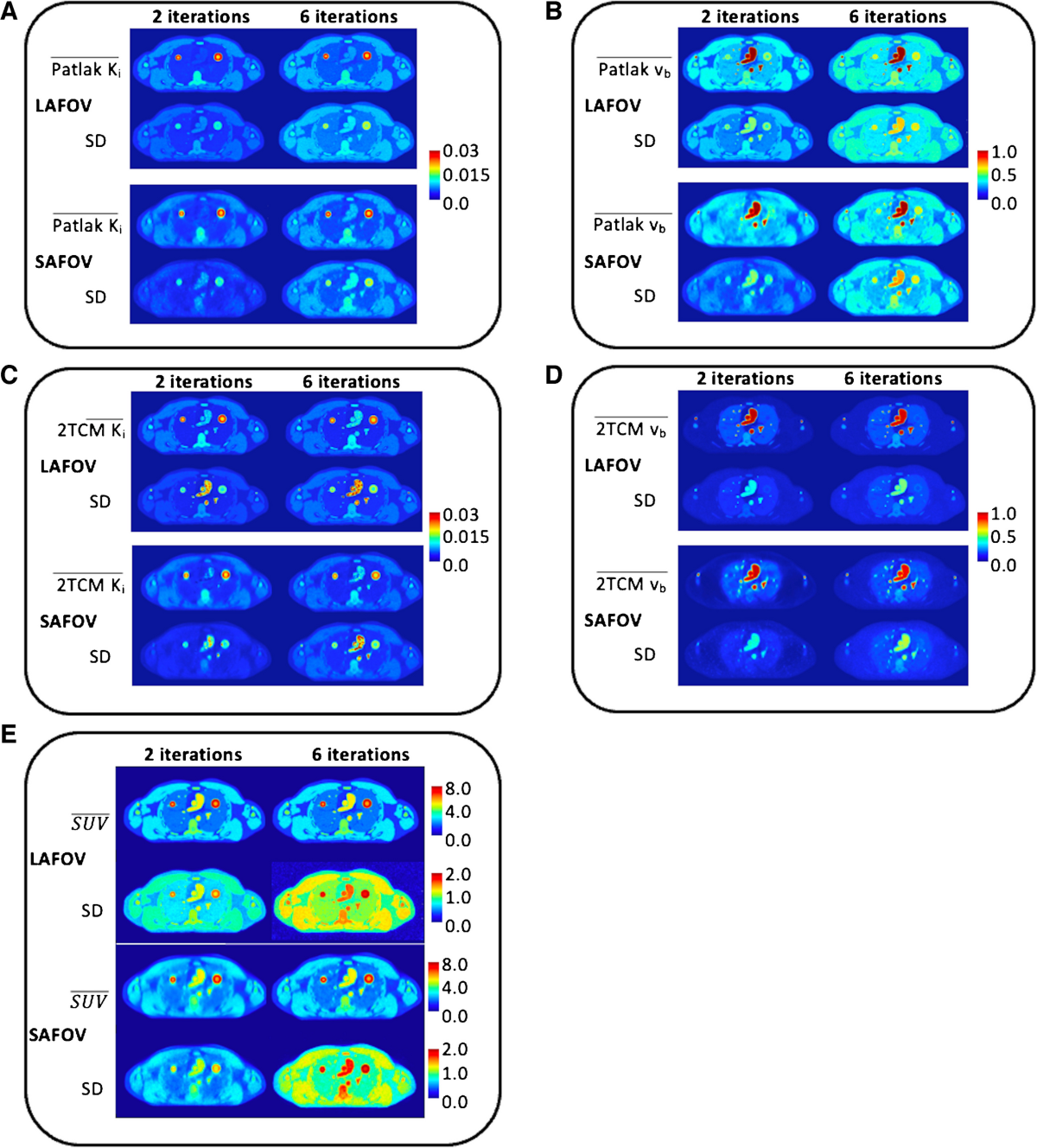
- Biases relative to ground truth varied by tissue, model, and configuration (Tables 3 and 4). For example, using 2 iterations, median bias reduction for LAFOV vs SAFOV was -37% for 2TCM parameters and -56% for Patlak parameters.
- Intrinsic biases of unprocessed SUV data were also quantified, ranging from 6.2±0.5% to 36.0±3.6% (SAFOV, 2it) and 0.3±0.1% to 8.5±2.3% (LAFOV, 2it).
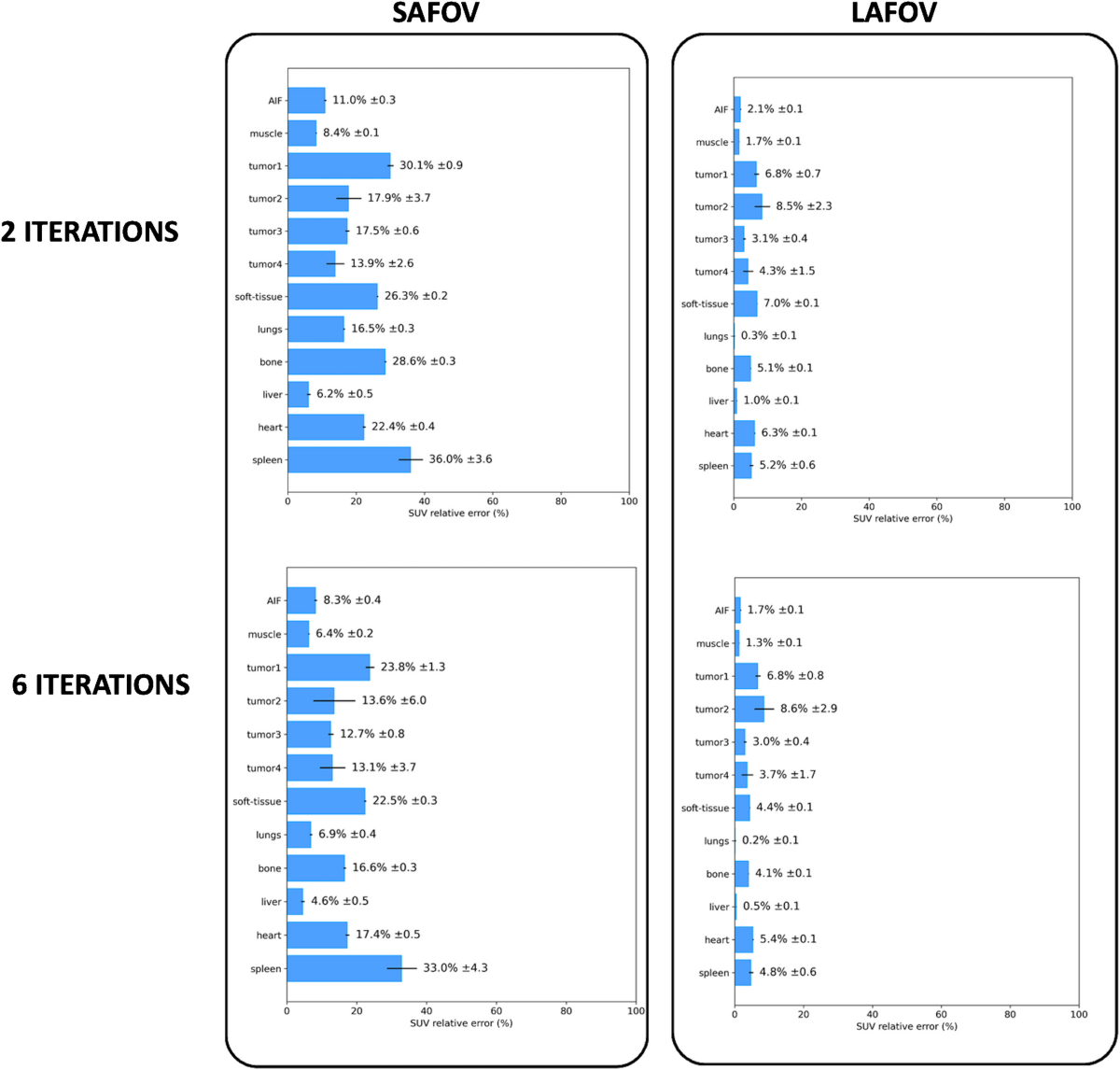
- Noise-bias trade-off plots demonstrated the superiority of the LAFOV configuration.
- Mean absolute kinetic-to-SUV CNR ratios exceeded 1 for Patlak Ki and vb maps in most tissue structures for both SAFOV and LAFOV configurations.
- The highest kinetic-to-SUV CNR ratio was observed in 2TCM k₃ maps within tumor regions (both center and border).
Discussions
The study effectively uses simulations to assess the reliability of PET KinetiX under controlled conditions, providing valuable insights into its performance compared to SUV and across different scanner types and reconstruction parameters. Limitations and suggestions for future work include:
- The simulations did not incorporate respiratory motion, which is a significant factor in thoracic imaging. Investigating the software's robustness to motion artifacts would increase clinical relevance.
- Only two OSEM iteration numbers (2 and 6) were tested. Exploring a wider range, potentially including post-filtering options common in clinical practice, could provide a more comprehensive understanding of the noise-bias trade-off.
- The study focused solely on 18F-FDG and irreversible models (Patlak, irreversible 2TCM). Evaluation with other tracers and reversible kinetic models (e.g., reversible 2TCM for tracers with k4 > 0) would broaden the software's applicability assessment.
- While the indirect kinetic modeling approach used by PET KinetiX offers universality, a comparison with direct reconstruction methods using the same simulated data could better quantify the potential trade-offs in bias and noise reduction.
- The sensitivity of the results to the definition and placement of the image-derived input function (IDIF) in the thoracic aorta could be further explored, as IDIF accuracy is critical for indirect modeling.
- Partial volume effects, especially at tissue interfaces and within the defined tumor border/center regions, likely influence parameter estimates. A discussion or analysis of this impact would be beneficial.
0
Subscribe to my newsletter
Read articles from Aldo Yang directly inside your inbox. Subscribe to the newsletter, and don't miss out.
Written by
