Article Review: Efficacy of combined targeted radionuclide therapy and immune checkpoint Inhibition in animal tumour models a systematic review and meta analysis of the literature
 Aldo Yang
Aldo Yang3 min read
Objectives
This systematic review and meta-analysis synthesizes preclinical evidence on combining targeted radionuclide therapy (TRT) and immune checkpoint inhibition (ICI).
- It demonstrates that combined TRT/ICI treatment significantly improves tumour growth control and survival compared to untreated controls, TRT monotherapy, and ICI monotherapy in animal tumour models.
- The findings provide preclinical proof-of-concept supporting the therapeutic potential of this combination strategy and its further evaluation in clinical trials.
Methodology
The study employed a systematic review and meta-analysis methodology.
- A systematic literature search was conducted in MEDLINE-PubMed and Embase-OVID databases.
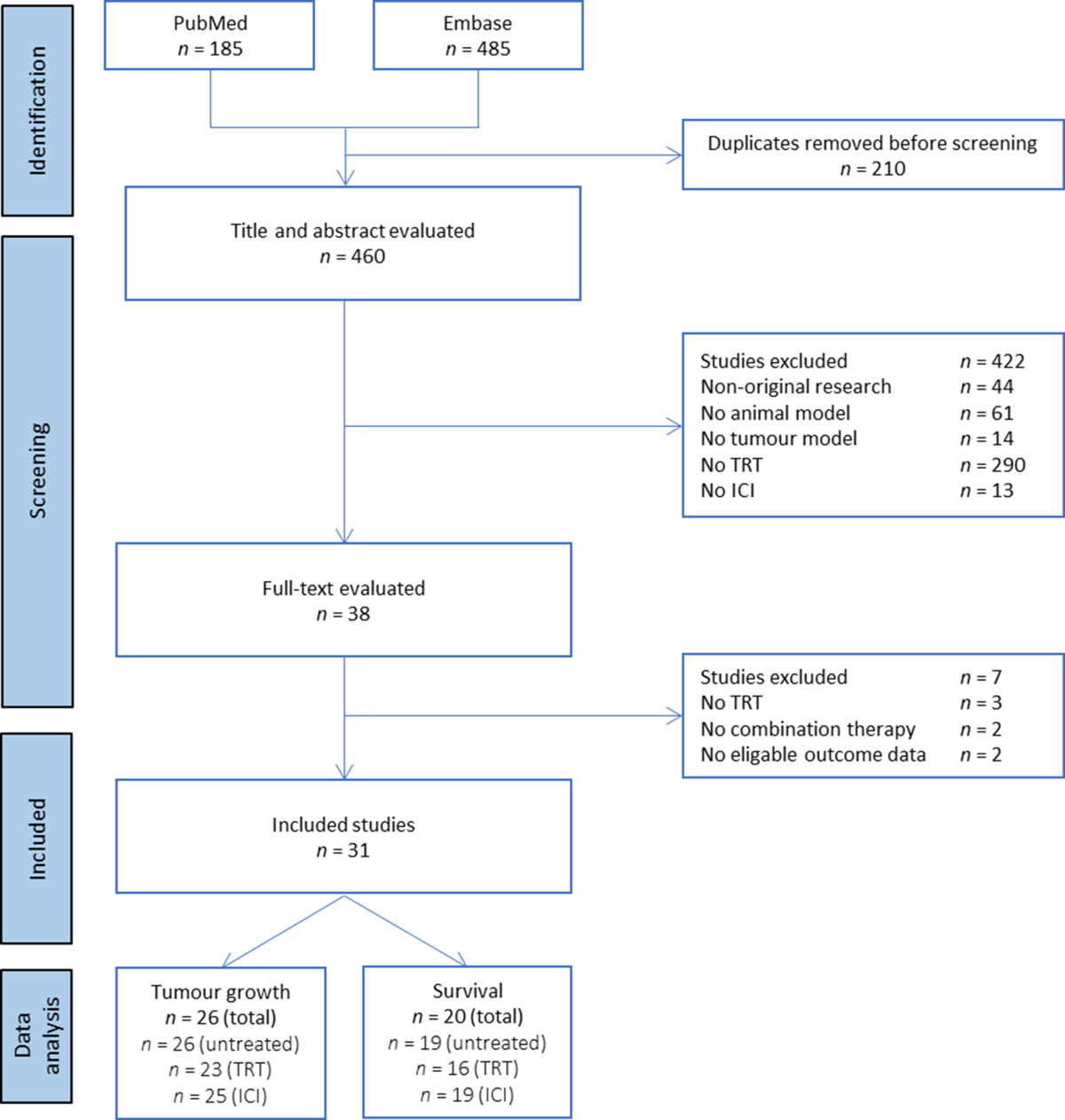
- Studies were selected based on predefined inclusion/exclusion criteria focusing on controlled animal experiments evaluating combined TRT/ICI.
- Data on tumour growth and survival were extracted. Tumour growth was analyzed using the normalized area under the curve (nAUC) ratio, and survival was analyzed using the restricted mean survival time (RMST) ratio.
- A random-effects model was used for meta-analysis to pool effect sizes (nAUC and RMST ratios) comparing combination therapy to control groups.
- Risk of bias in included studies was assessed using an adapted SYRCLE tool.
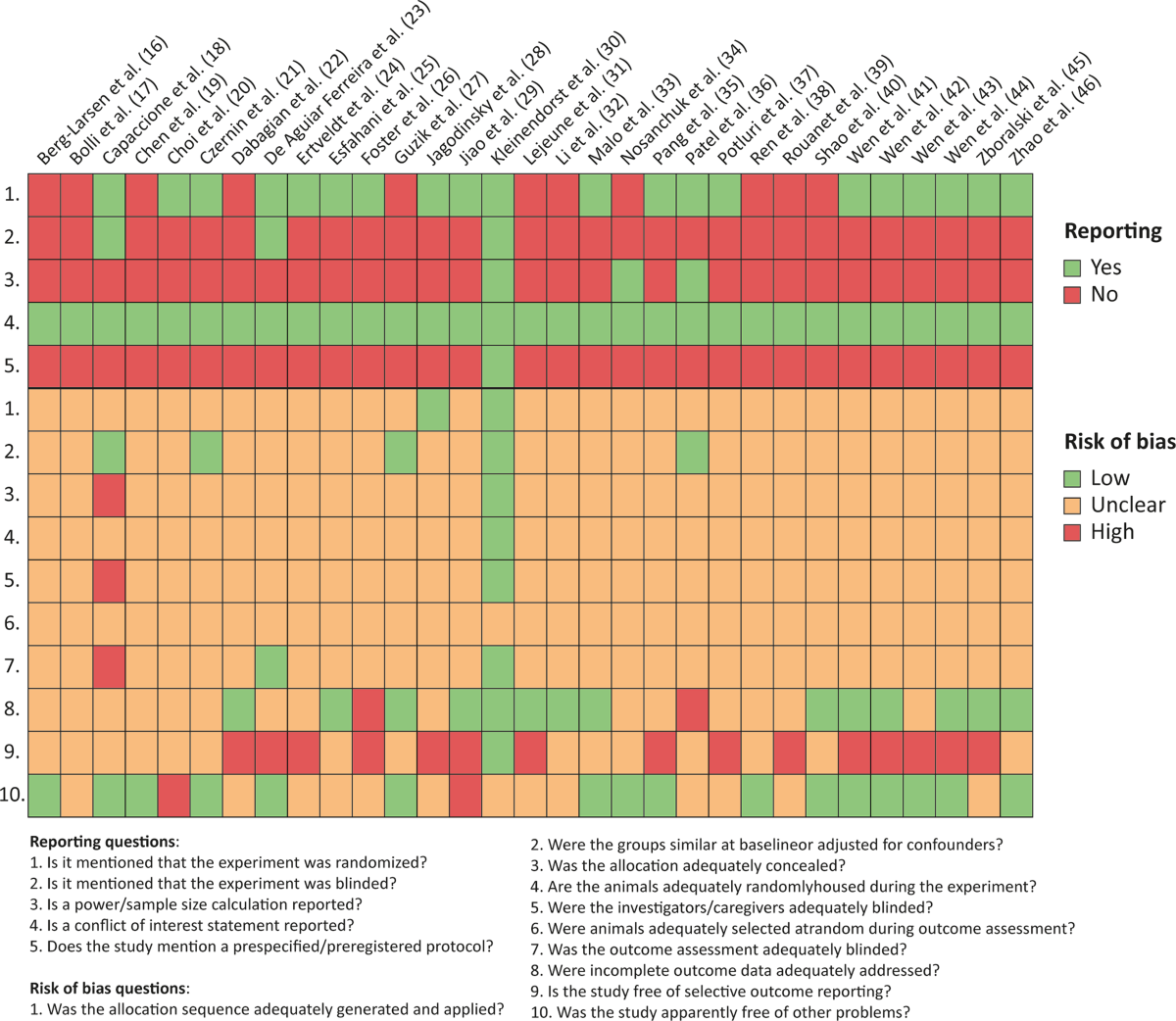
- Heterogeneity was assessed using the I2 statistic, and subgroup analyses were performed for predefined potential moderators.
- Sensitivity analysis and publication bias assessment (funnel plots, trim-and-fill) were conducted.
Results
The meta-analysis provides quantitative support for the efficacy of combined TRT/ICI:
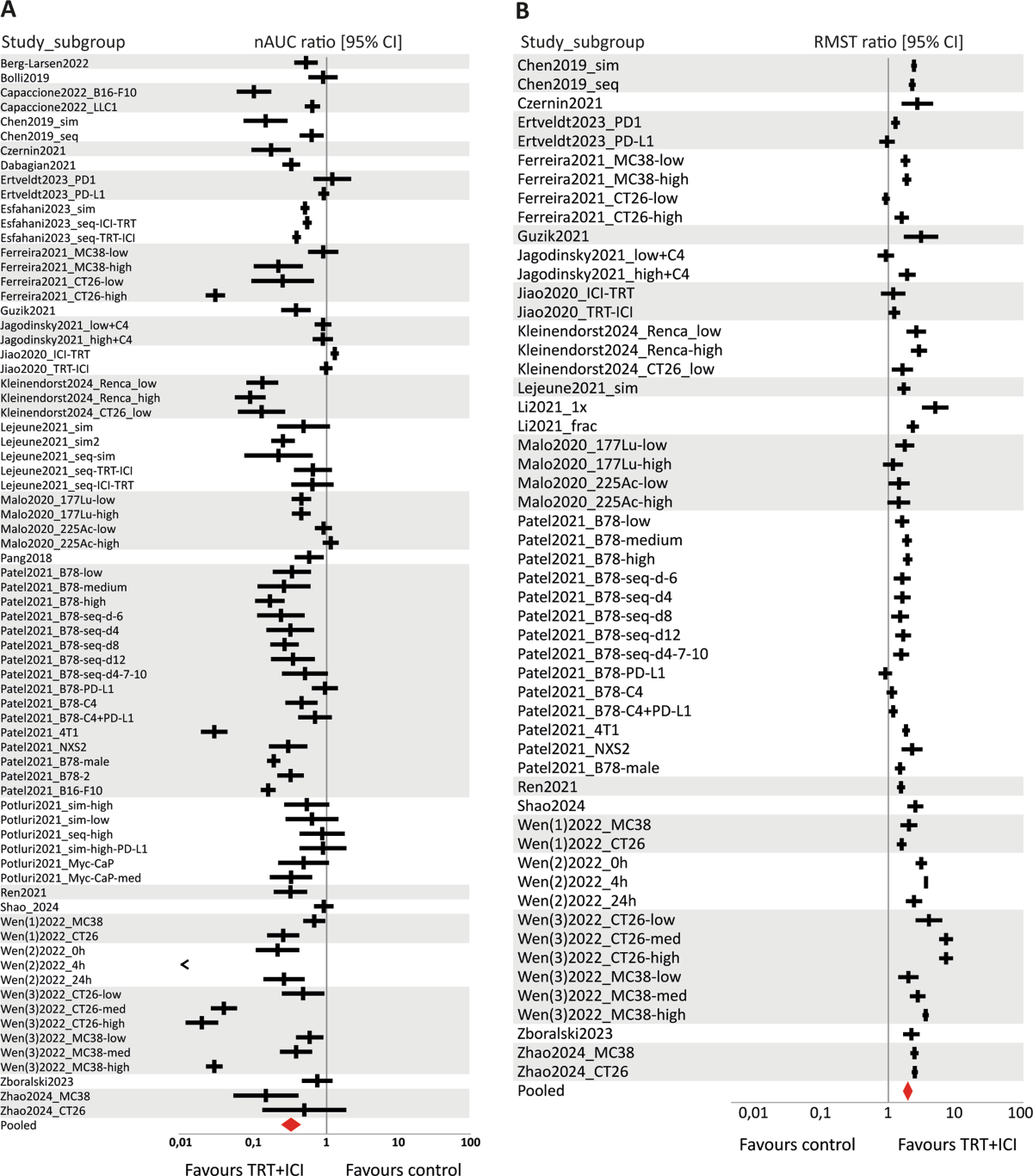
- Compared to untreated controls, combination therapy significantly improved survival (RMST ratio: 1.96, 95% CI [1.72–2.23]) and reduced tumour growth (nAUC ratio: 0.32, 95% CI [0.25–0.42]).
- Compared to TRT monotherapy, combination therapy significantly improved survival (RMST ratio: 1.44, 95% CI [1.34–1.55]) and reduced tumour growth (nAUC ratio: 0.49, 95% CI [0.41–0.59]).
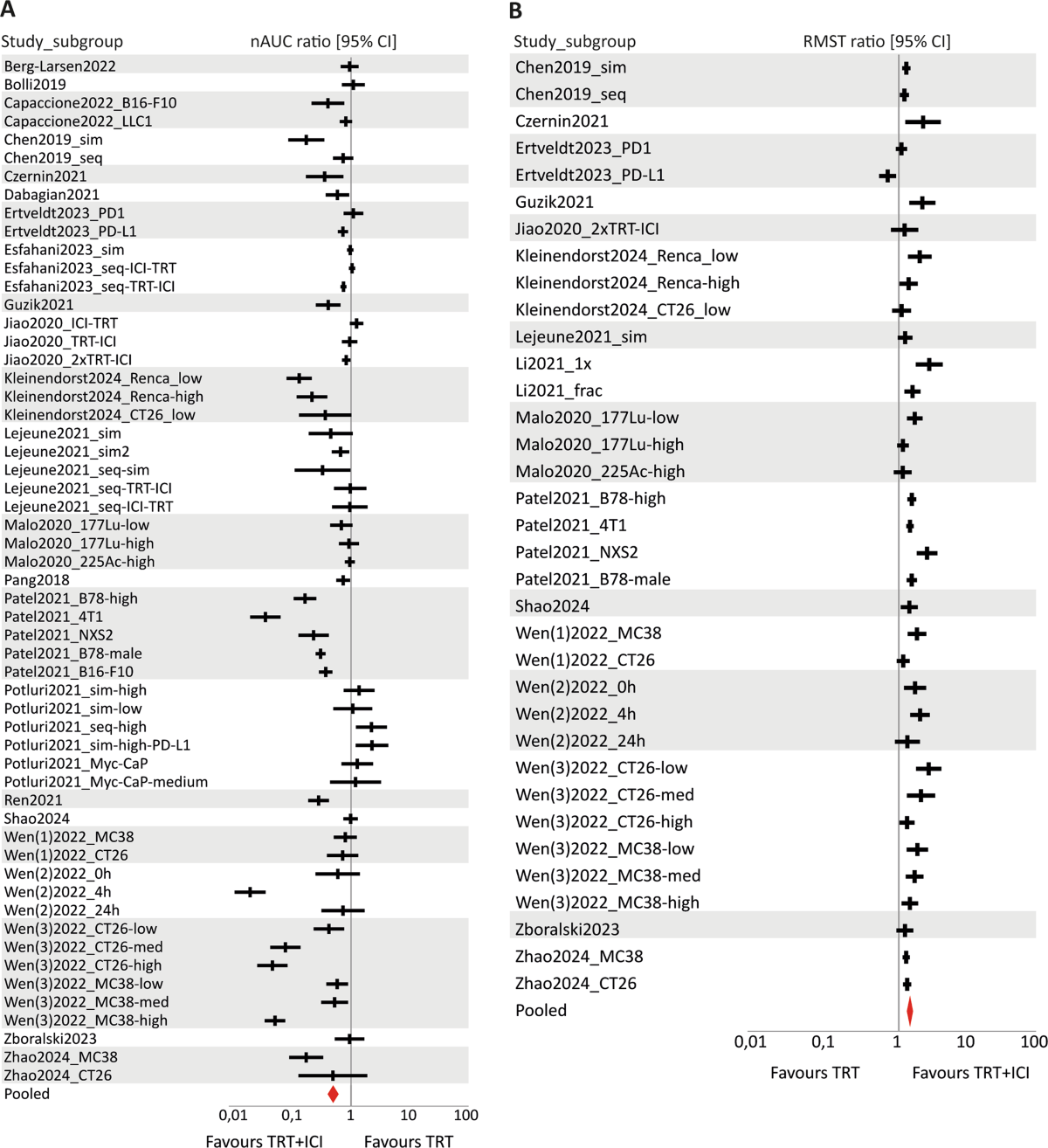
- Compared to ICI monotherapy, combination therapy significantly improved survival (RMST ratio: 1.54, 95% CI [1.38–1.72]) and reduced tumour growth (nAUC ratio: 0.41, 95% CI [0.31–0.55]).
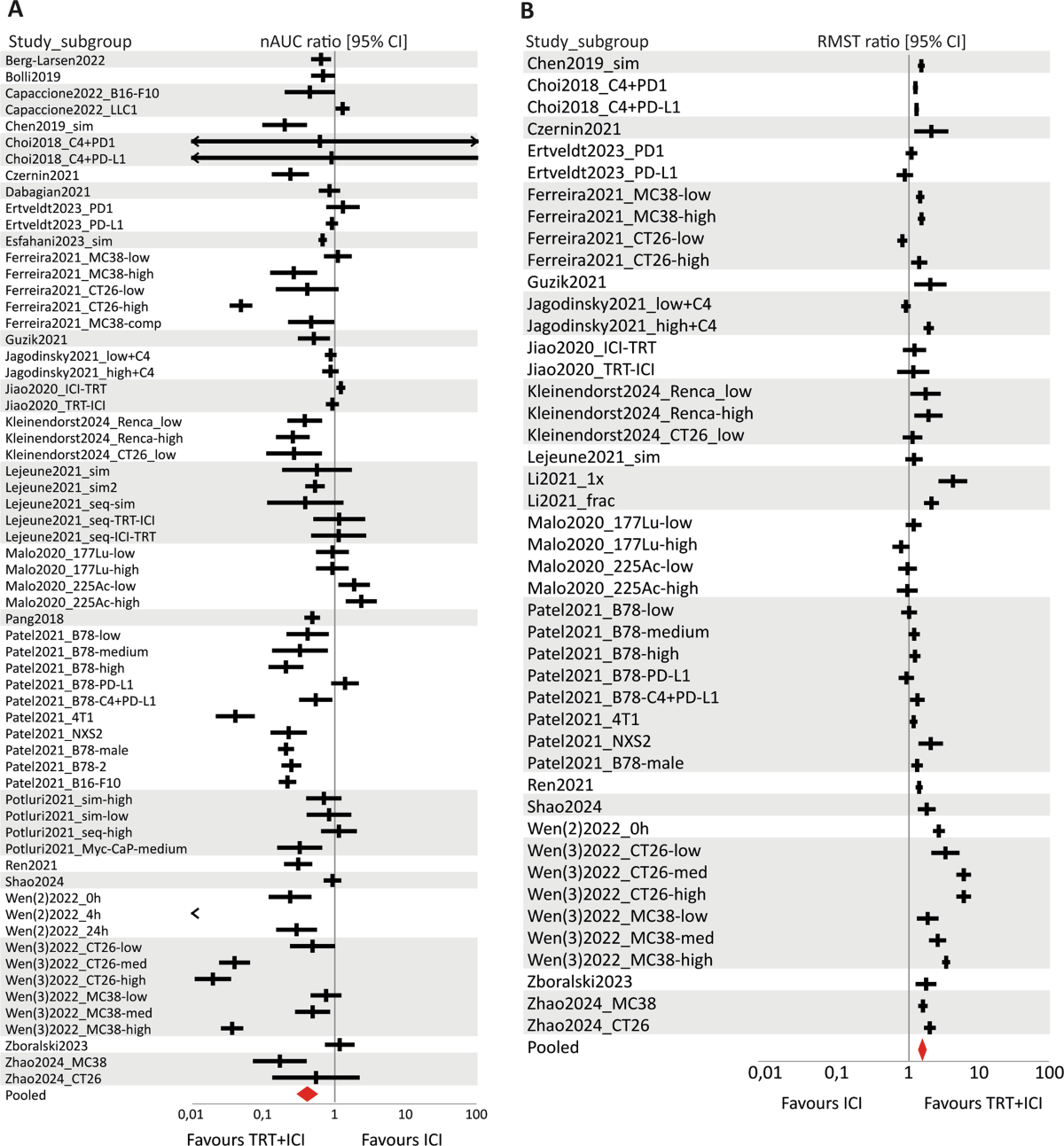
- All pooled effect sizes were statistically significant, favouring the combination treatment.
- High between-study heterogeneity was observed (I2 = 76.7–98.2%).
- Subgroup analysis suggested the type of ICI (aPD-L1 vs. aPD-1) might be a moderator of treatment efficacy for tumour growth outcomes, with aPD-L1 combinations appearing more effective.
Discussions
This systematic review provides valuable preclinical evidence supporting TRT/ICI combination therapy. However, several limitations impact the interpretation and translation of findings:
- Reporting Quality: Many included studies suffer from poor reporting (e.g., lack of randomization details, blinding, sample size calculation), leading to an 'unclear' risk of bias for many domains. This hinders a robust assessment of study quality.
- Heterogeneity: High heterogeneity across studies (animal models, TRT targets/doses, ICI types, schedules) limits the ability to draw firm conclusions about optimal treatment parameters.
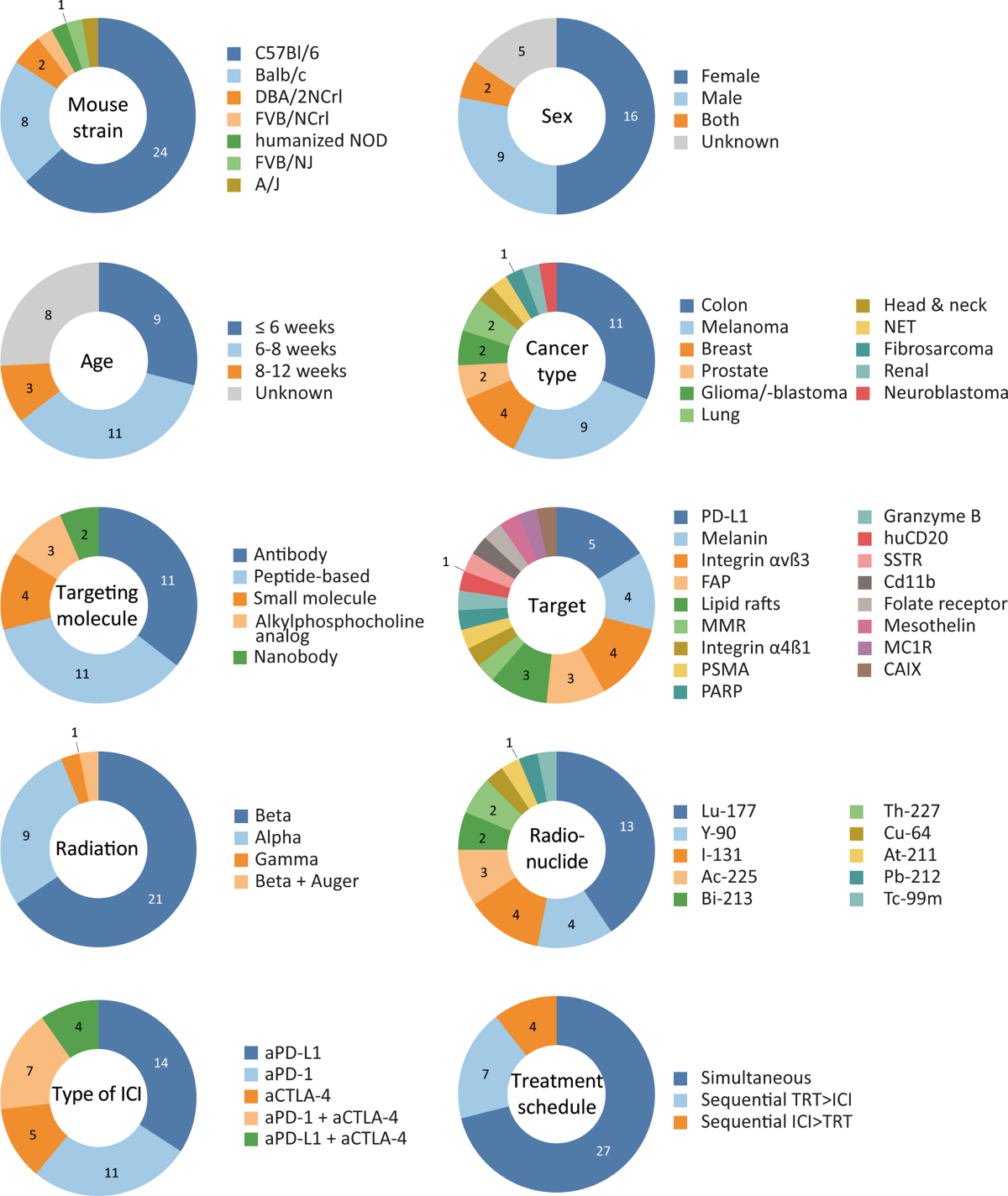
- Dosimetry: A major gap is the lack of reported tumour-absorbed dose in most studies (24 out of 31), preventing dose-response analysis and hindering clinical translation of dosing strategies.
- Model Relevance: The predominance of young (<8 weeks) mice and subcutaneous syngeneic models may not fully reflect human tumour microenvironments or immune responses. Future studies should consider more complex models (orthotopic, metastatic, GEMMs) and appropriately aged animals.
- Subgroup Analysis Limitations: Insufficient data prevented subgroup analyses for key factors like alpha vs. beta emitters or treatment sequencing, although differences between aPD-1 and aPD-L1 combinations were suggested.
- Recommendations: Future preclinical work should prioritize standardized reporting (e.g., ARRIVE guidelines, TRT radiobiology reporting recommendations), protocol preregistration, inclusion of dosimetry, use of clinically relevant models, and systematic investigation of parameters like dose, radionuclide type, ICI target, and sequencing.
0
Subscribe to my newsletter
Read articles from Aldo Yang directly inside your inbox. Subscribe to the newsletter, and don't miss out.
Written by
