Article Review: DICOM header editing between possibilities and regulatory bounds 40 years of DICOM standard
 Aldo Yang
Aldo YangObjectives
This letter discusses the persistent challenges and complexities surrounding Digital Imaging and Communications in Medicine (DICOM) header editing, 40 years after the standard's establishment.
- It highlights discrepancies arising from intentional modifications (e.g., for research, changing modality tags like NM to PT for SPECT/US fusion) and unintentional modifications (e.g., during data transfer between vendor platforms).
- It points out the conflict between the need for flexibility in research (e.g., using analysis tools across modalities) and the rigid constraints imposed by DICOM standards and regulatory frameworks.
- It calls for improved intermodality compatibility from manufacturers, a more flexible regulatory approach, and collaboration between stakeholders to establish guidelines for metadata editing.
- It suggests a blockchain-inspired approach to enhance traceability and data integrity for DICOM metadata.
Methodology
The letter focuses on the technical and procedural aspects of DICOM header management.
- It describes how DICOM metadata (tags like Study Instance UID (0020,000D) or Modality (0008,0060)) can be modified intentionally using DICOM editors for specific purposes (e.g., enabling SPECT data use in tools designed for PT/CT) or unintentionally altered/deleted during data transfer between systems (e.g., PACS from different vendors).
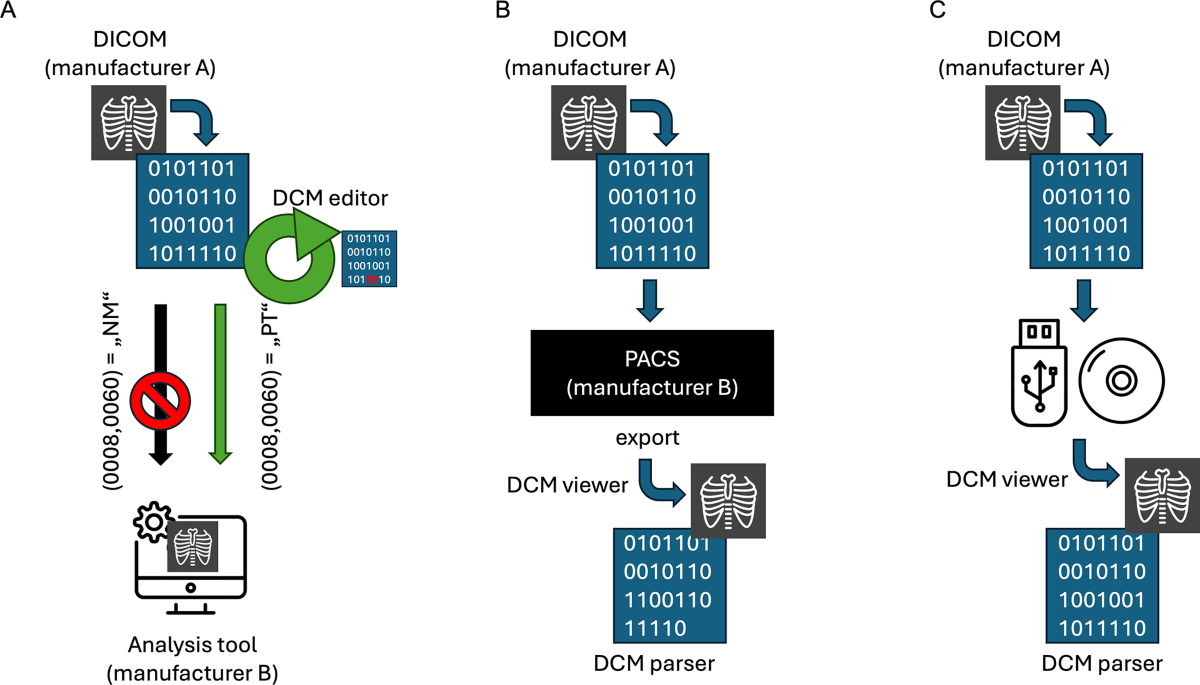
- It notes challenges in nuclear medicine with the 'NM' tag during SPECT data processing.
- It mentions that DICOM parsing and editing require expertise and can be time-consuming.
- It proposes enhancing traceability beyond existing methods like DICOM Digital Signatures by using a blockchain-based system to create a tamper-proof record of metadata changes, referencing existing work [3].
Results
The letter does not present primary experimental data to quantify the prevalence or impact of DICOM header issues. Its claims are supported by:
- Citing examples of intentional modification needs (e.g., changing SPECT modality tag to PT for US fusion, referencing Freesmeyer et al. [2]).
- Illustrating types of modifications (intentional, unintentional, none) with a diagram.
- Referencing literature on related solutions (e.g., blockchain for tracking access [3]).
- General observations about inconsistencies between manufacturer implementations and the challenges faced in research and clinical practice over 40 years of the standard.
Discussions
The letter effectively raises awareness about a significant and persistent issue in medical imaging: the challenges and risks associated with DICOM header inconsistencies and modifications.
- The distinction between intentional and unintentional modifications, along with the provided examples (e.g., SPECT tag change for fusion), clearly illustrates the problem's dimensions.
- The call for collaboration, improved manufacturer practices, and regulatory flexibility is well-justified.
- The suggestion of a blockchain-inspired approach for traceability is forward-thinking, although its practical implementation within diverse hospital IT ecosystems warrants further discussion regarding feasibility, scalability, and cost-effectiveness.
- A limitation is the lack of quantitative data within the letter itself regarding the frequency or specific clinical/research consequences of these header issues across different institutions or modalities. Further investigation, perhaps through surveys or multi-center data analysis, could strengthen the argument for the proposed changes.
Reference: DICOM header editing between possibilities and regulatory bounds 40 years of DICOM standard
Subscribe to my newsletter
Read articles from Aldo Yang directly inside your inbox. Subscribe to the newsletter, and don't miss out.
Written by
