Why Pharma’s Top Performers Rely on These 7 CDM Best Practices
 Sarah R. Weiss
Sarah R. Weiss
Achieving strong ROI in the pharmaceutical world isn’t just about adopting the latest technology — it’s about mastering the data that powers it. The most successful pharma companies know that behind every breakthrough is a well-oiled clinical data management (CDM) system.
These industry leaders aren’t just collecting data — they’re using it strategically to streamline trials, cut operational costs, and make faster, more informed decisions.
In this post, we’ll break down the 7 best practices in clinical data management that top pharma performers rely on to consistently deliver results — and share ways to visualize each step to make implementation smoother for your teams.
1. Streamline Data Collection and Integration
In clinical data management, data comes from various sources like EHRs, clinical trial management systems, and lab records. Managing this data manually is inefficient and prone to error.
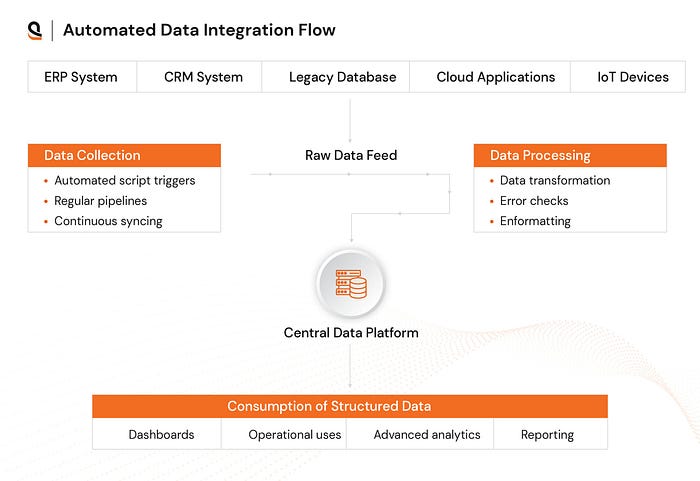
Unified integration through automated data collection ensures that data flows freely between departments, eliminating delays and data-entry errors. The result? Faster decision-making and more accurate insights at every stage of a trial.
2. Automated Data Validation & Cleaning for Faster Clinical Insights
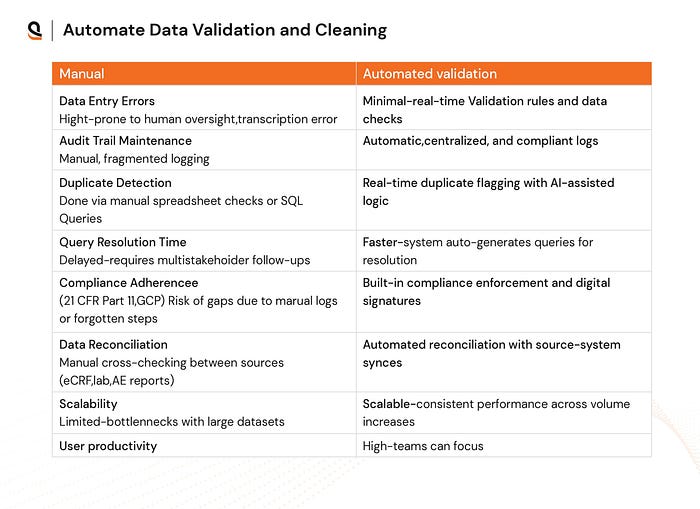
Data validation and cleaning are often manual processes, leading to delays and human errors. Without proper validation, the data used for decision-making could be flawed, risking costly setbacks.
Automating validation processes enables quick identification of inaccuracies, outliers, or missing values, ensuring that only clean data is used. This reduces error rates and speeds up data processing.
3. Real-Time Data Analytics: Fueling Faster, Smarter Outcomes
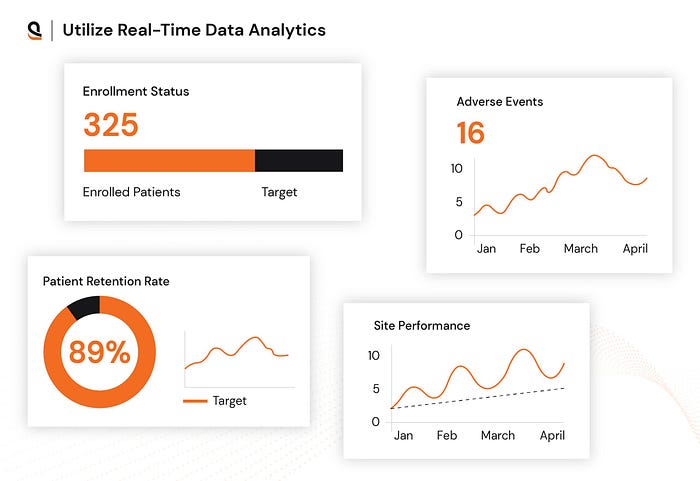
Clinical trials generate vast amounts of data that need to be analyzed in real time for effective decision-making. Without immediate access to data, adjustments may be delayed, leading to inefficiencies and increased costs.
Real-time analytics enable quick identification of trends, issues, and deviations in clinical trials, allowing teams to adapt rapidly and make data-driven decisions.
4. Use Predictive Analytics for Risk Management
Predictive analytics leverages historical data and trends to forecast potential risks such as adverse events, delays, or trial failures.
Predictive tools allow you to anticipate and manage risks before they disrupt trials, preventing costly mistakes and resource wastage.
Subscribe to my newsletter
Read articles from Sarah R. Weiss directly inside your inbox. Subscribe to the newsletter, and don't miss out.
Written by

Sarah R. Weiss
Sarah R. Weiss
I share insights on Software Development, Data Science, and Machine Learning services. Let's explore technology together!